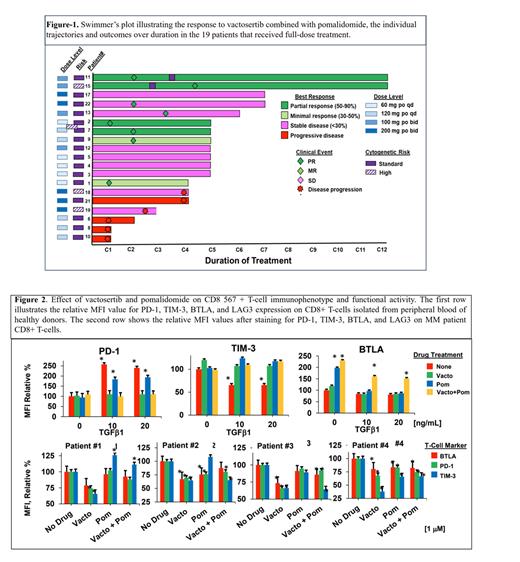
The prognosis for Multiple Myeloma (MM) patients (pts) has improved substantially over the past two decades with the success of immunomodulatory drugs (IMiDs), monoclonal antibodies, chimeric antigen receptor (CAR) T-cells and bispecific T-cell engagers (BiTEs) that broke the circulus vitiosus of tumor-induced immunosuppression to reengage the immune system. Functional blockade of the transforming growth factor-beta (TGF) signaling pathway improves the efficacy of cytotoxic and immune therapies, therefore we investigator the impact of TGF-inhibition in an steroid free regimn. Methods: We conducted a phase 1b study to determine the safety, efficacy, and maximal tolerated dose (200 mg po bid) of the potent, orally-available TGF type I receptor kinase inhibitor vactosertib in relapsed and/or refractory multiple myeloma (RRMM) pts who had received -2 lines of chemoimmunotherapy in combination with standard dosage of Pomalidomide without any corticosteroids. Results: Twenty one pts were enrolled in the phase 1b dose escalation cohort and received vactosertib plus pomalidomide as indicated (Fig. 1A). Pts' characteristics are described in Table 1The summary of grade 1-4 adverse events is shown (Table 2). A single grade 4 elevated bilirubin (5%) and a single grade 4 elevated lipase (5%) were observed as well as four grade 4 events of decreased neutrophils (19%). Four pts demonstrated a PR, two had a MR, nine had SD and four had disease progression (Fig. 1B). The median time to response for all pts and the responders eight weeks and median duration of response was ~12 weeks. The best overall response for individual pts to treatment is shown (Fig. 1C). Clinical activity was observed across all four dose levels (Table 3). To probe the tumor intrinsic anti-myeloma activity of vactosertib, we first determined the relative effect of vactosertib compared to the IMiDs pomalidomide and lenalidomide (Fig. 2A, B). The effect of vactosertib was greater than that observed with either lenalidomide or pomalidomide. MMCLs and patient CD138 + cells were then treated with vactosertib in combination with pomalidomide, and importantly, vactosertib with pomalidomide synergistically reduced myeloma viability (Fig. 3A). We next probed the systemic, tumor extrinsic effects of vactosertib by analyzing cytokines immune and tumor cells present within patient BM samples. (Fig. 4A). (Fig. 4B). Therefore, we explored the immunophenotype of CD8 + T-cells isolated from patient BM prior to and at the EOT. The relative percentage of T-cells that expressed the immunosuppressive markers programmed cell death-1 (PD-1), T-cell immunoglobulin and mucin domain-containing protein 3 (TIM3), B and T lymphocyte attenuator (BTLA), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) was reduced following treatment relative to their expression prior to treatment (Fig. 4C). Results indicated that vactosertib and pomalidomide treatment in vivo reduced PD-1, TIM3, BTLA and CTLA-4 expression on patient CD8 + T-cells. Next, CD8 + T-cells were isolated from patient peripheral blood and cultured in the presence of TGF-1, vactosertib, pomalidomide, or vactosertib plus pomalidomide (Fig. 5B). Treatment of patient CD8 + T-cells with vactosertib decreased PD-1 expression up to 40% and decreased TIM3 expression up to 65%. Pomalidomide alone did not significantly reduce PD-1, TIM3, BTLA, or LAG-3 on patient CD8 + T-cells. Vactosertib and pomalidomide also reduced TIM3 levels on CD8 + T-cells from most pts. (Fig. 5C, D). (Fig. 5 G, H). Results indicated that vactosertib or pomalidomide did not increase CD138 + cell death (Fig. 5I). Conclusion: Vactosertib combined with pomalidomide was well-tolerated at all doses, had a manageable adverse event profile and induced durable responses with 80% progression-free survival (PFS-6) at 6 months, Vactosertib reduced TGF- in patient bone marrow and suppressed PD-1 expression on CD8 + T-cells and lead to reduction of PD-L1/PD-L2 expression on CD138 + cells and enhanced autologous T-cell cytotoxicity. Taken together, our results support the safety and efficacy of vactosertib to treat RRMM and revealed that vactosertib modulates the T-cell immunophenotype and reinvigorates T-cell fitness.
Disclosures
Malek:Cumberland Inc.: Research Funding; Sanofi Inc.: Consultancy; Medpacto Inc.: Research Funding; BMS: Consultancy; Karyopharm: Speakers Bureau; BMS: Speakers Bureau; Amgen: Honoraria. Kim:Medpacto Inc.: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal